Reverse Deactivated Radial Polymerisations (RDRP)
Advances in polymer synthesis provide a means to fine-tune the properties of macromolecules by varying the size, shape, chemical composition and architecture. The invention of commonly used reversible-deactivation radical polymerisations (RDRPs) including RAFT (reversible addition−fragmentation chain-transfer) polymerisation, ATRP (atom-transfer radical polymerisation) and NMP (nitroxide-mediated polymerisation) began over 20 years ago and has significantly increased the chemoselectivity of reactions; thus, RDRP allows the use of more complex and functional monomers. However, the main disadvantage of RDRPs is that extremely high molecular weights are difficult to achieve with narrow dispersity because early termination reactions may occur.
Two methods of RDRP that I used during my Honours year were ATRP and Single-electron transfer living-radical polymerisation (SET-LRP).
ATRP is a method of controlled polymerisation that uses a copper catalyst. In the polymerisation, a Cu(I) halide promotes the formation of free radicals (Pn*) via halide abstraction and simultaneously undergoes a redox reaction with itself to produce Cu(II) halide (major) or Cu(0) (minor). Reduction of Cu(II) to Cu(I) produces a dormant species (Pn-x). An amine ligand, for example 1,1,4,7,10,10-hexamethyltriethylenetetramine (HMTETA), is used in this reaction to solubilise the copper catalyst in organic solvents and to increase catalytic activity.
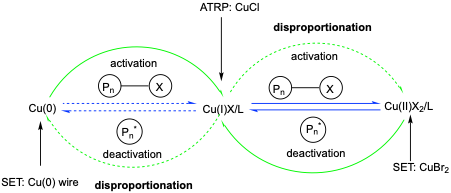
Figure caption. Major reactions in SET-LRP (green) and ATRP (blue). The dotted arrows in the figure represent minor reactions for both methods of polymerisation.
SET-LRP is a deviant method of ATRP that uses copper wire, Cu(0), to promote the formation of free radicals. The copper wire reacts with an alkyl bromide to initiate chain growth; ultimately undergoing a redox reaction, from Cu(0) to Cu(I). The Cu(I) species is short-lived and reacts with itself to form Cu(II) halide and Cu(0), referred to as disproportionation in Figure. An amine-containing ligand, for example Me6TREN, further facilitates deactivation of radicals by preferentially binding to Cu(II).