Tacticity
During the polymerisation of a chiral molecule (see [[Chirality]] for more information), the monomer will attach to a propagating chain in either an ordered or disordered fashion - that is, all the monomers will form right-handed polymers, left-handed polymers or random. For the former, either right-handed or left-handed polymers are termed isotactic, while the latter is termed atactic. In a situation that the individual units on the polymer chain are alternating: both left and right-handed (i.e. a racemic mixture) but in an ordered fashion, this is termed syndiotactic.
Tacticity vs. Crystallinity The configuration of each unit along the polymer chain will influence the physical properties of the polymer chain, as the arrangement and regularity of the chain will determine the degree of crystallinity. In a random/atactic polymer, the structure is mostly amorphous, while a highly regular/syndiotactic or atactic polymer will be crystalline/semi-crystalline.
Tacticity is therefore an important structural parameter to control, as a more crystalline polymer will be a lot harder and less flexible. It will also have discrete melting and crystallisation temperatures. In contrast, an amorphous polymer will be more rubber like with more room to move or flow. Instead of it having a discrete melting and crystallisation temperature, it will have a "glass-transition" temperature. This is the point that the material turns from a glass-like substance to a more flexible, rubbery-like substance.
100% syndio/isotacticity is very difficult to achieve. Also, considering that the instruments that measure the degree of tacticity usually exhibit errors ranging from 1-5%, atmost, 95% tacticity is achievable. Thus, most polymers consist of amorphous regions.
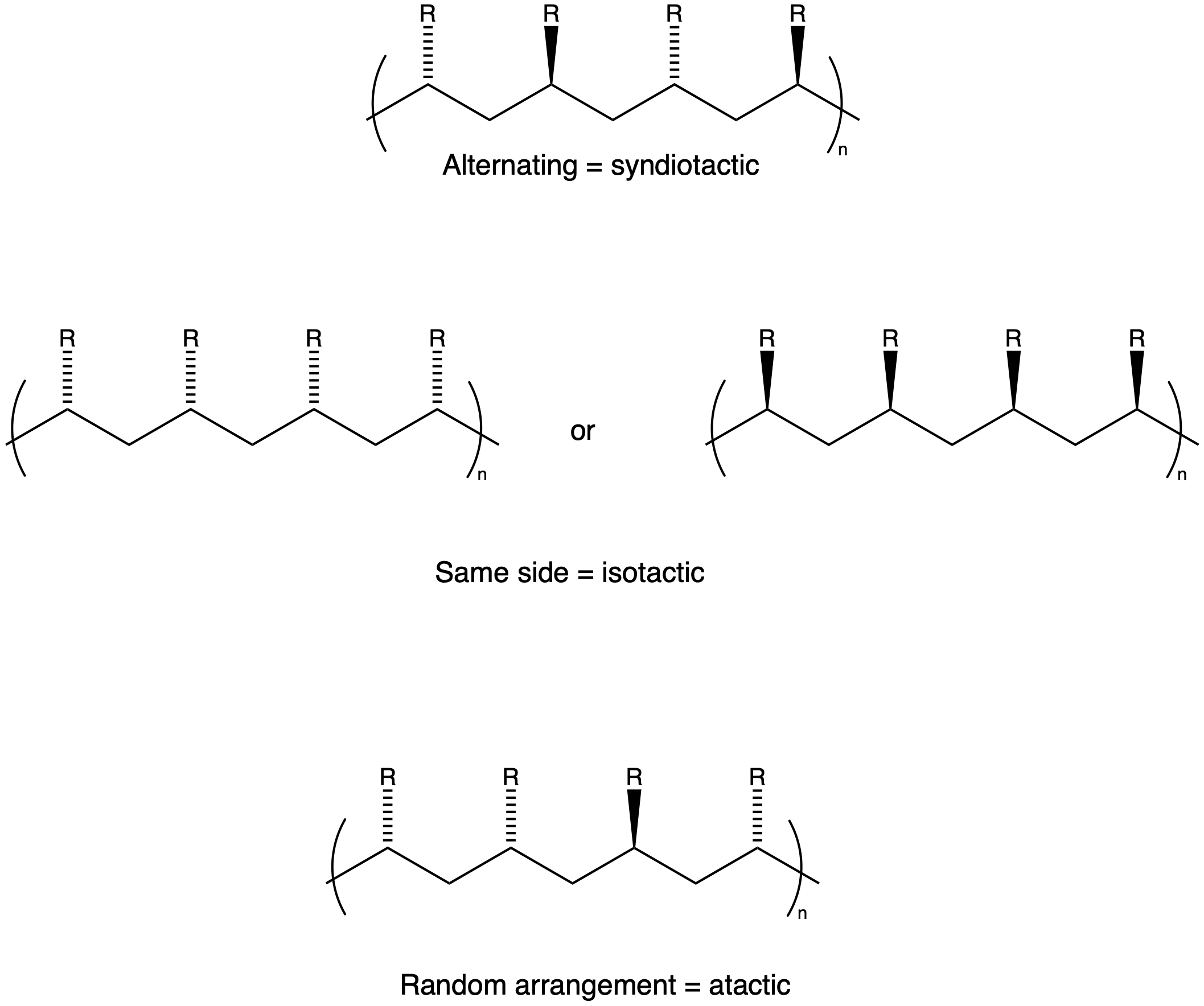 Figure 1. Syndiotactic vs isotactic vs atactic.
Figure 1. Syndiotactic vs isotactic vs atactic.
In semi-conducting polymers, tacticity is not often assigned. This is usually because semi-conducting polymers are very difficult to characterise and not usually accessible by methods such as proton NMR. Thus, in this field of research, [[Regioregularity]] is often used as a descriptor for the arrangement of monomers along the polymer chain.
While there is a need to improve the regularity of semi-conducting polymers to form more ordered polymers with enhanced electronic properties, amorphous regions in a semi-crystalline polymer are important for faster charge transport. Perhaps we shouldn't be focusing on achieving 100% crystalline films, but instead how to better order the amorphous domains within a semi-crystalline polymer to facilitate charge transport.