Synthesising a conducting polymer with a biomolecular dopant
This is something that I have explored throughout my PhD: using an ionic biomaterial to polymerise EDOT. Well others also have this idea.
19.5.23
Today I attended a seminar by Prof. Simon Moulton, who is a professor of biomedical engineering at Swinburn University. He spoke about some of his work with Lubricin, where his PhD student used the negatively charged components of lubricin to electrochemically polymerise poly(pyrrole) (PPy).
Lubricin is a natural bottlebrush polymer that provides lubrication in the synovial joints. When it is casted onto a surface like gold, the adhesive ends tether to the surface. This leaves exposed regions that are negatively charged.
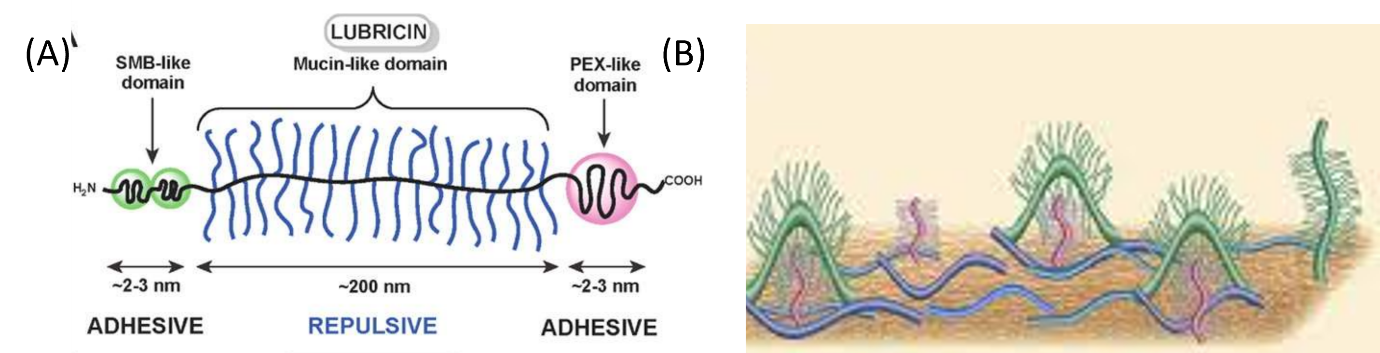 Fig 1. Macromolecular structure of lubricin
Fig 1. Macromolecular structure of lubricin
By introducing pyrrole (Py) in water and applying an electrochemical potential, pyrrole polymerises and uses the negatively charged sulphate groups of lubricin to dope the backbone and allow polymerisation to proceed.
This was monitored through quartz microbalance, where they monitored the changes in mass as the number of cyclic voltammetry deposition cycles increase. They observed that the Ppy flattened the lubricin and the resulting films were very thin. I did ask about the UV-vis properties and what the films looked like. He said that he hadn't investigated that but what he described about the appearance of the film seemed to be similar to observations of my materials in chapter 6.