Gluten intolerance
Bread is subjectively the best thing to exist on Earth. Do you agree? Well about 5-10% of you will not agree. This is because gluten intolerance is a fairly prevalent allergy. Celiac disease is the name given to a gluten intolerance, which is diagnosed in about 1% of people wordwide. People with this disease experience vomiting, bloating, abdominal pain, numbness in the hands and feet among many other symptoms, in response to gluten ingestion. Why does this occur?
Celiac disease is commonly inherited through the genes. One genetic marker of celiac is the human leukocyte antigen (HLA)-DQ2 and HLA-DQ8 genotype. About 30% of people will express this genotype, however, only 1-3% of people will develop the disease. These two genes present on Antigen-Presenting Cells (APCs) to produce an immune response when exposed to peptides from Gliadin.
As I explained in my previous bread post, gluten is composed of gliadin, which is the harmful component to people with celiac. Gliadin is difficult to digest, even in healthy humans, due to the presence of prolamins (a group of plant storage proteins). These proteins are generally hydrophobic (or water-hating), with the central region composed mostly of glutamine and proline amino acids. As a result, gliaden will pass into the intestinal barrier. The presence of gliadin in the small intestine produces an inflammtory response, where cells will release tissue transamidase (tTG), often the reason why we would experience bloating in response to breads. tTg expression is increased in celliac sufferers.
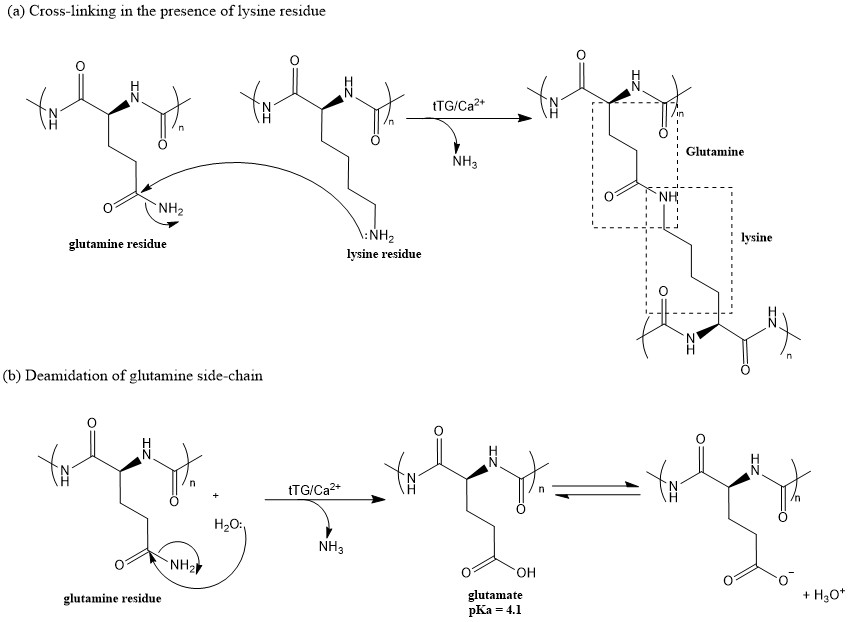 |
|---|
| Fig 1. (a) Crosslinking of glutamine and lysine to form a high molecular weight polymer or (b) deamidation of glutamine to form glutamate which is negatively charged at physiological pH. Both reactions are catalysed by tTG. |
Once released from cells, tTG crosslinks a protein with a glutamine residue (in this case, the gliadin) to a protein with a lysine group, via a peptide bond. This cross-linking is an important step that leads to insoluble protein aggregates, which can be cleared from the body. However, it can also convert glutamine to glutamic acid in a process called deamidation.
Deamidation of gliadin produces negatively charged peptides at physiological pH (7.4-7.6), which then has a high affinity for HLA-DQ2 and HLA-DQ8. The binding of the glutamic acid to the HLA proteins, results in T-cell stimulation, followed by release of proinflammatory cytokines. This inflammatory response leads to the destruction of the intestinal villi, which are responsible for absorbing nutrients.In addition, people with celliac disease, antibodies are produced in response to the tTG release, further invoking the limitation in gliadin digestion and thus, increasing the prevalence of inflammation.
References
- Sha, X., et al., The prolamins, from structure, property, to the function in encapsulation and delivery of bioactive compounds. Food Hydrocolloids, 2024. 149: p. 109508.
- Aboulaghras, S., et al., Pathophysiology and immunogenetics of celiac disease. Clinica Chimica Acta, 2022. 528: p. 74-83.1.
- Lerner, A. and C. Benzvi, "Let Food Be Thy Medicine": Gluten and Potential Role in Neurodegeneration. Cells, 2021. 10(4).
- Parzanese, I., et al., Celiac disease: From pathophysiology to treatment. World J Gastrointest Pathophysiol, 2017. 8(2): p. 27-38.
- Lorand, L. and R.M. Graham, Transglutaminases: crosslinking enzymes with pleiotropic functions. Nature Reviews Molecular Cell Biology, 2003. 4(2): p. 140-156.